Kanpur, 21 December 2022: Prof. Mahendra Verma and research scholar Soumyadeep Chatterjee from the Department of Physics, IIT Kanpur, have discovered that 2D Euler flow—flow with zero viscosity--evolves from disorder to order. The study involving analytical arguments and accurate numerical simulations provides useful insights that can help the manner in which scientists approach important fundamental topics such as order to disorder evolution, Second Law of Thermodynamics and thermalization, the process through which physical processes reach thermal equilibrium.
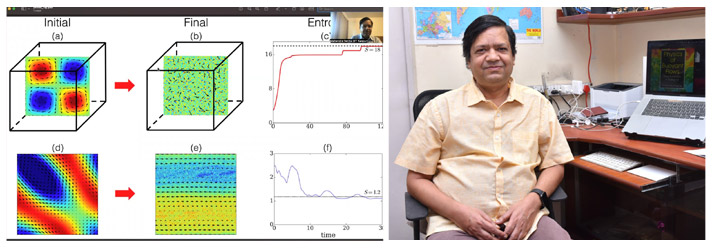
The study, “Hydrodynamic Entropy and Emergence of Order in Two-dimensional Euler Turbulence” published in Physical Review Fluids journal finds that 2D Euler flow evolves from order to disorder and that the system is out of equilibrium with an interesting energy exchange among the flow structures so as to violate the detailed energy balance. In fact, there is an inverse cascade of energy, which is responsible for the nonequilibrium behaviour of 2D Euler turbulence.
As per laws of Thermodynamics, the thermodynamic entropy of the Euler flow remains constant. The researchers, Prof. Verma and Mr. Chatterjee, proposed “hydrodynamic entropy” as a measure of order in a multiscale and nonequilibrium system such as hydrodynamic and astrophysical systems, and employed it to Euler turbulence. They have shown that the hydrodynamic entropy of 2D Euler flow decreases with time during its approach to the asymptotic state. The duo has also found that the final state of the flow depends critically on the initial condition.
However, since the second law as the thermodynamic entropy remains constant during the evolution, the decrease in hydrodynamic entropy in 2D Euler flow is not a violation of the Second Law of Thermodynamics. The findings illustrate that the isolated dynamical system may evolve from disorder to order at macroscopic scales and that there is a need to be cautious of general claims on the “evolution from order to disorder” in any system. Based on their findings, Prof. Verma and Mr. Chatterjee believe that a similar evolution may occur in self-gravitating systems.
The hydrodynamic entropy proposed in the study, as per the researchers, may prove to be a useful tool for quantifying order in biological, hydrodynamic, astrophysical, ecological, and economic systems.
Reference: Verma, Mahendra, Chatterjee, Soumyadeep. "Hydrodynamic Entropy and Emergence of Order in Two-dimensional Euler Turbulence" Physical Review Fluids. Volume 7. November 2022. Article No: 114608.
About IIT Kanpur:
Indian Institute of Technology (IIT) Kanpur was established on 2nd November 1959 by an Act of Parliament. The institute has a sprawling campus spread over 1055 acres with large pool of academic and research resources spanning across 19 departments, 22 centres, and 3 Interdisciplinary programs in engineering, science, design, humanities, and management disciplines with 540 full-time faculty members and approximately 9000 students. In addition to formal undergraduate and postgraduate courses, the institute has been active in research and development in areas of value to both industry and government.
For more information, visit https://www.iitk.ac.in.











