-
Recovery of Lignosulfonates from
Spent Sulphite Liquor
Separation and purification of Lignosulfonates,
present in spent sulphite liquor, has been carried
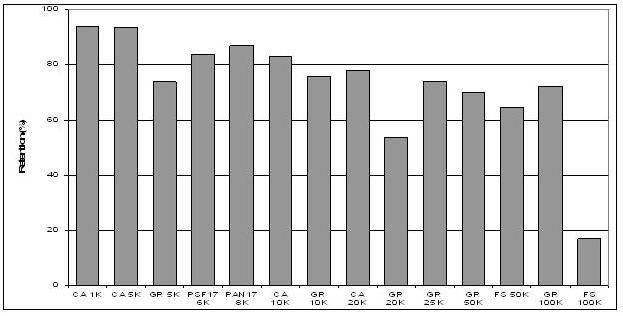
out through a Dia-filtration operation. Around
80-85% recovery is obtained. The technology
development is complete and ready for pilot plant
trial. Results of molecular mass distribution of
lignosulphates have shown that variable size
distribution may be obtained by using different
cut-off size of membranes.
* P. K. Bhattacharya, R. K. Todi, M. Tiwari, C.
Bhattacharjee, S. Bhattacharjee, S. Dutta, “Studies
on UF of spent sulphite liquor (SSL) using various
membranes for the recovery of lignosulphonates“,
Desalination, 174, 287 – 297 (2005).
-
Micellar Enhanced Ultrafiltration (MEUF)
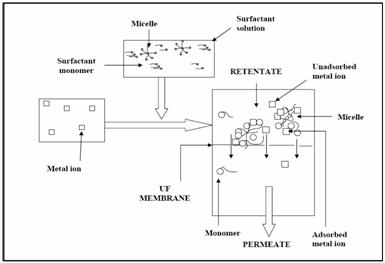
In MEUF process, the surfactant having charge
opposite to target ions, is added to the effluent
stream containing the metal ions at a concentration
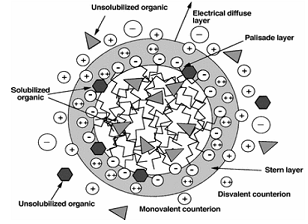
greater than the critical micellar concentration
(CMC), so that they form aggregates of around 50–150
of monomer molecules, called micelles. Therefore, a
large fraction of the metal ions get
electrostatically attached to the micelle surface.
Retention of such metal ions attached to the
micelles is possible if the resulting solution is
passed through an ultrafilter, having pore size
smaller than the micelle diameter. The recovery and
the reuse of surfactant are of utmost importance
from economical point of view. Several studies were
done for the removal of metal ion and organic
solutes under aqueous medium through MEUF under both
stirred and unstirred conditions using batch cells.
Bonding or solubilizations of these solutes in
surfactant micelles were also experimentally
ascertained. Mathematical models were also
developed.
* M. Syamal, S. De and P. K. Bhattacharya, “Phenol
solubilization by cetyl pyridinium chloride micelles
in micellar enhanced ultrafiltration“, J.
Membrane Sci., 137, 99-107 (1997).
* S. Ralph Jadhav, N. Verma, A. Sharma and P. K.
Bhattacharya, “Flux and retention analysis during
micellar enhanced ultrafiltration for the removal of
phenol and aniline“, Separ. Purif. Technol., 24,
541-557 (2001).
* Gargi Ghosh and Prashant K. Bhattacharya, "Hexavalent
chromium ion removal through micellar enhanced
ultrafiltration", Chem. Eng. J.: Environ. Chem.
Eng., 119, 45 - 53 (2006).
-
Hydrazine Hydrate Separation through
Pervaporation
Hydrazine is an important inorganic chemical that
upon combustion produces nitrogen and water, unlike
hydrocarbon fuels producing carbon dioxide and
water. Hydrazine is less flammable and less volatile
than hydrocarbon fuels and hence it is used as a
component in jet fuels because it produces a large
amount of heat when burned. It finds active
applications in rocket propulsion; however, in
anhydrous hydrazine form. By ordinary distillation,
64 wt.% of hydrazine is produced. Therefore, removal
of water from hydrate state to produce anhydrous
hydrazine is essential. Conventional separation
technique, like distillation forms an azeotrope with
water at 71.5 wt% of hydrazine. Further, hydrazine
and water, being highly polar substances (surface
tension values very close) impart strong hydrogen
bonding between them. Therefore, pervaporation
processes* is recommended to seek dehydration.
* S. V. Satyanarayana, P. K. Bhattacharya,
“Pervaporation of Hydrazine Hydrate: Separation
characteristics of membranes with hydrophilic to
hydrophobic behaviour“, J. Membrane Sci., 238,
103 -115 (2004).
* Nazish Hoda, Satyanarayana V. Suggala and Prashant
K. Bhattacharya, “Pervaporation of Hydrazine – Water
through Hollow Fiber Module: Modeling and
Simulation“, Computers Chem. Eng., 30 (2),
202-214 (2005).
* Mrinal Kanti Mandal, Sukalyan Dutta and P. K.
Bhattacharya, “Characterization of Blended Polymeric
Membranes for Pervaporation of Hydrazine Hydrate”,
Chem. Eng. J., 138, 10-19 (2008).
-
Ultrafiltration of Sugar Cane Juice
for recovery of Sugar
Sugar industry is one of the largest agro-based
industries in India; however, suffer from many
problems. There is need to investigate the energy
saving and technically efficient alternative
processes. Application of the integrated membrane
technology is has been attempted (processes like RO,
UF, MF and ED. Such processes may offer certain
advantages in clarification and concentration of
multi-component solutions and suspensions like sugar
cane juice. However, the membrane systems pose
problems like decline of flux over time which
prevents widespread applicability of such processes
commercially. Several studies* were made
accordingly.
* A. D. Sarode, N. Verma and P. K. Bhattacharya,
“Analysis of retention and flux decline during
ultrafiltration of limed sugarcane (clarified)
juice“, Chem. Eng. Commun., 188, 179-206
(2001).
* U. V. S. RamGopal, N. Verma and P. K.
Bhattacharya, “Analysis of flux decline during
ultrafiltration of sugarcane juice (limed) using
cross-flow cell“, Can. J. Chem. Eng., 80(1),
105-115 (2002).
* P. K. Bhattacharya, Shilpi Agarwal, S. De and
U.V.S. RamaGopal, “Ultrafiltration of sugar cane
juice for recovery of sugar: analysis of flux and
retention“, Separ. Purif. Technol., 21,
247-259 (2001).
-
Enzymatic (immobilized) Membrane
reactor
Enzymatic hydrolysis of lactose mainly yields
glucose and galactose. In addition galacto-oligosaccharides
(GOS) are also formed by transgalactosylation
activity of enzyme from the same biochemical
reaction. GOS is a very beneficial functional
component for human. GOS, also recognized as
prebiotics consists of a number of oligosaccharides
which are linked with different b-glycosidic bonds,
depending on enzyme source. Work is going on in this
direction.
-
Prehydrolysis Liquor Treatment to
Disposal Level
Treatment of prehydrolysis liquor was done
biologically using sacchromyces cerevisiae,
zymomonus mobilis and torulla utilis as strains,
which convert sugar into yeast. High percentages (75
to 90%) of reductions in sugars, COD, BOD and total
dissolved solids were observed. Modeling was done to
predict the rate equations for all the cases.
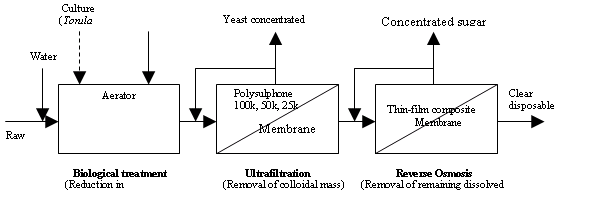
Further to this biological treatment,
ultra-centrifuging was done to separate out the
formed yeast. The clarified biologically treated
liquor was then subjected to UF/ RO to obtain the
desired level of quality of permeate for disposal.
The membrane operation becomes extremely simpler
because of the depletion of micro-solutes (by bio
means) as the osmotic pressure of the feed solution
gets drastically reduced.
* R. L. Rath, C. Bhattacharjee, Shikha Jain, P. K.
Bhattacharya, “Treatment of prehydrolysis liquor
from pulp mill using biological route followed by
Reverse Osmosis“, Chem. Eng. Technol., 28 (10),
1201-1211 (2005).
* R. Jayan, C. Bhattacharjee and P. K. Bhattacharya,
“A combined biological and membrane based treatment
of prehydrolysis liquor from pulp mill“, Separ.
Purif. Technol., 45 (2), 119-130 (2005).