|
Fourier Transform Infrared Spectrometer (FTIR) |
||||
|
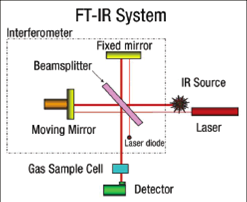
FTIR is most useful for identifying chemicals that are either organic or inorganic. It can be utilized to quantitate some components of an unknown mixture and for the analysis of solids, liquids, and gases. The term Fourier Transform Infrared Spectroscopy (FTIR) refers to a development in the manner in which the data is collected and converted from an interference pattern to a spectrum. It is a powerful tool for identifying types of chemical bonds in a molecule by producing an infrared absorption spectrum that is like a molecular "fingerprint". The wavelength of light absorbed is characteristic of the chemical bond as can be seen in this annotated spectrum. The FT-IR (Bruker-Tensor) (Figure 1) was established in the Core Lab (201D) in 2008 with the DRDO grant of the Institute. Basic Principle:Molecular bonds vibrate at various frequencies depending on the elements and the type of bonds. For any given bond, there are several specific frequencies at which it can vibrate. According to quantum mechanics, these frequencies correspond to the ground state (lowest frequency) and several excited states (higher frequencies). One way to cause the frequency of a molecular vibration to increase is to excite the bond by having it absorb light energy. For any given transition between two states the light energy (determined by the wavelength) must exactly equal the difference in the energy between the ground state and the first excited state (Figure 2).Unique Features: High sensitivity performance with the permanently aligned, high cube corner interferometer, customizable workspaces, hyperspectral imaging,the rmogravimetric coupling, high throughput screening devices and easy measurement mode. |
||||
|
Location: |
||||
|
Department of Chemical Engineering,Core Lab 201D, |
||||
|
Contact: |
||||
|
Prof. Nishith Verma |
||||