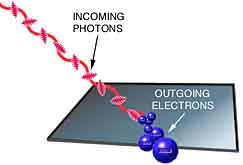
 It's been determined experimentally that when light shines on
a metal surface, the surface emits electrons. For example, you
can start a current in a circuit just by shining a light on a
metal plate. Why do you think this happens?
It's been determined experimentally that when light shines on
a metal surface, the surface emits electrons. For example, you
can start a current in a circuit just by shining a light on a
metal plate. Why do you think this happens? |
|
 Well...we
were saying earlier that light is made up of electromagnetic waves,
and that the waves carry energy. So if a wave of light hit an electron
in one of the atoms in the metal, it might transfer enough energy
to knock the electron out of its atom.
Well...we
were saying earlier that light is made up of electromagnetic waves,
and that the waves carry energy. So if a wave of light hit an electron
in one of the atoms in the metal, it might transfer enough energy
to knock the electron out of its atom.
 Okay. Now, if light is indeed composed of waves, as you suggest...
Okay. Now, if light is indeed composed of waves, as you suggest...
|
 |
 What do you mean, "if light is composed of waves"? Is there
another option?
What do you mean, "if light is composed of waves"? Is there
another option? |
 Historically,
light has sometimes been viewed as a particle rather than a
wave; Newton, for example, thought of light this way. The particle
view was pretty much discredited with Young's double slit experiment,
which made things look as though light had to be a wave. But in the
early 20th century, some physicists--Einstein, for one--began to examine
the particle view of light again. Einstein noted that careful experiments
involving the photoelectric effect could show whether light consists
of particles or waves.
Historically,
light has sometimes been viewed as a particle rather than a
wave; Newton, for example, thought of light this way. The particle
view was pretty much discredited with Young's double slit experiment,
which made things look as though light had to be a wave. But in the
early 20th century, some physicists--Einstein, for one--began to examine
the particle view of light again. Einstein noted that careful experiments
involving the photoelectric effect could show whether light consists
of particles or waves.
 How?
It seems to me that the photoelectric effect would still occur no
matter which view is correct. Either way, the light would carry energy,
so it would be able to knock electrons around.
How?
It seems to me that the photoelectric effect would still occur no
matter which view is correct. Either way, the light would carry energy,
so it would be able to knock electrons around.
 Yes, you're
right--but the details of the photoelectric effect come out
differently depending on whether light consists of particles or waves.
If it's waves, the energy contained in one of those waves should depend
only on its amplitude--that is, on the intensity of the light. Other
factors, like the frequency, should make no difference. So, for example,
red light and ultraviolet light of the same intensity should knock
out the same number of electrons, and the maximum kinetic energy of
both sets of electrons should also be the same. Decrease the intensity,
and you should get fewer electrons, flying out more slowly; if the
light is too faint, you shouldn't get any electrons at all, no matter
what frequency you're using.
Yes, you're
right--but the details of the photoelectric effect come out
differently depending on whether light consists of particles or waves.
If it's waves, the energy contained in one of those waves should depend
only on its amplitude--that is, on the intensity of the light. Other
factors, like the frequency, should make no difference. So, for example,
red light and ultraviolet light of the same intensity should knock
out the same number of electrons, and the maximum kinetic energy of
both sets of electrons should also be the same. Decrease the intensity,
and you should get fewer electrons, flying out more slowly; if the
light is too faint, you shouldn't get any electrons at all, no matter
what frequency you're using.
 That sounds
reasonable enough to me. How would the effect change if you assume
that light is made of particles?
That sounds
reasonable enough to me. How would the effect change if you assume
that light is made of particles?
 I should
give you some background information on this, first. It all began
with some work on radiation by Max Planck... In 1900, Max Planck was
working on the problem of how the radiation an object emits is related
to its temperature. He came up with a formula that agreed very closely
with experimental data, but the formula only made sense if he assumed
that the energy of a vibrating molecule was quantized--that is, it
could only take on certain values.The energy would have to be proportional
to the frequency of vibration, and it seemed to come in little "chunks"
of the frequency multiplied by a certain constant. This constant came
to be known as Planck's constant, or h, and it has the value =6.626*10
to the power of-34
I should
give you some background information on this, first. It all began
with some work on radiation by Max Planck... In 1900, Max Planck was
working on the problem of how the radiation an object emits is related
to its temperature. He came up with a formula that agreed very closely
with experimental data, but the formula only made sense if he assumed
that the energy of a vibrating molecule was quantized--that is, it
could only take on certain values.The energy would have to be proportional
to the frequency of vibration, and it seemed to come in little "chunks"
of the frequency multiplied by a certain constant. This constant came
to be known as Planck's constant, or h, and it has the value =6.626*10
to the power of-34
 That's
a pretty small constant.
That's
a pretty small constant.
 Yes, but
it was an extremely radical idea to suggest that energy could only
come in discrete lumps, even if the lumps were very small. Planck
actually didn't realize how revolutionary his work was at the time;
he thought he was just fudging the math to come up with the "right
answer," and was convinced that someone else would come up with a
better explanation for his formula.
Yes, but
it was an extremely radical idea to suggest that energy could only
come in discrete lumps, even if the lumps were very small. Planck
actually didn't realize how revolutionary his work was at the time;
he thought he was just fudging the math to come up with the "right
answer," and was convinced that someone else would come up with a
better explanation for his formula.
 I
guess Einstein must have taken him seriously, though.
I
guess Einstein must have taken him seriously, though.
 Quite
seriously. Based on Planck's work, Einstein proposed that light also
delivers its energy in chunks; light would then consist of little
particles, or quanta, called photons, each with an energy
of Planck's constant times its frequency.
Quite
seriously. Based on Planck's work, Einstein proposed that light also
delivers its energy in chunks; light would then consist of little
particles, or quanta, called photons, each with an energy
of Planck's constant times its frequency.
 In that
case, the frequency of the light would make a difference in
the photoelectric effect.
In that
case, the frequency of the light would make a difference in
the photoelectric effect.
 Exactly.
Higher-frequency photons have more energy, so they should make the
electrons come flying out faster; thus, switching to light with the
same intensity but a higher frequency should increase the maximum
kinetic energy of the emitted electrons. If you leave the frequency
the same but crank up the intensity, more electrons should
come out (because there are more photons to hit them), but they won't
come out any faster, because each individual photon still has
the same energy.
Exactly.
Higher-frequency photons have more energy, so they should make the
electrons come flying out faster; thus, switching to light with the
same intensity but a higher frequency should increase the maximum
kinetic energy of the emitted electrons. If you leave the frequency
the same but crank up the intensity, more electrons should
come out (because there are more photons to hit them), but they won't
come out any faster, because each individual photon still has
the same energy.
 And if
the frequency is low enough, then none of the photons will have enough
energy to knock an electron out of an atom. So if you use really low-frequency
light, you shouldn't get any electrons, no matter how high
the intensity is. Whereas if you use a high frequency, you should
still knock out some electrons even if the intensity is very low.
And if
the frequency is low enough, then none of the photons will have enough
energy to knock an electron out of an atom. So if you use really low-frequency
light, you shouldn't get any electrons, no matter how high
the intensity is. Whereas if you use a high frequency, you should
still knock out some electrons even if the intensity is very low.
 Quite right.
Therefore, with a few simple measurements, the photoelectric effect
would seem to be able to tell us whether light is in fact made up
of particles or waves.
Quite right.
Therefore, with a few simple measurements, the photoelectric effect
would seem to be able to tell us whether light is in fact made up
of particles or waves.
 So did someone do the experiment? Which way did it turn out?
So did someone do the experiment? Which way did it turn out?
 In 1913-1914,
R.A. Millikan did a series of extremely careful experiments involving
the photoelectric effect. He found that all of his results agreed
exactly with Einstein's predictions about photons, not with the wave
theory. Einstein actually won the Nobel Prize for his work on the
photoelectric effect, not for his more famous theory of relativity.
In 1913-1914,
R.A. Millikan did a series of extremely careful experiments involving
the photoelectric effect. He found that all of his results agreed
exactly with Einstein's predictions about photons, not with the wave
theory. Einstein actually won the Nobel Prize for his work on the
photoelectric effect, not for his more famous theory of relativity.